
The atom might resemble a tiny solar system, with a massive,

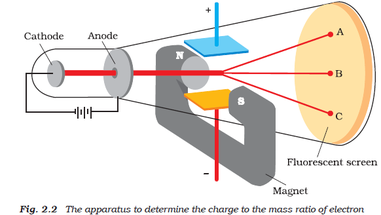
Using aĭifferent kind of particle beam, Rutherford found evidence that This theory was struck down by Thomson's own former student, Ernest Rutherford. cathode rays are streams of negatively charged ions all the mass of an atom is essentially in the nucleus the e/m of electrons is much greater than the e/m of. If Thomson had found the single building block of all atoms, how could atoms be built up out of these corpuscles? Thomson proposed a model, sometimes called the "plum pudding" or "raisin cake" model, in which thousands of tiny, negatively charged corpuscles swarm inside a sort of cloud of massless positive charge. Heories about the atom proliferated in the wake of Thomson's 1897 work. Real understanding required many more experiments over later Experiments by Thomson, Lenard, and others through the crucial year of 1897 were not enough to settle the uncertainties. Radually scientists accepted Thomson's first and second hypotheses, although with some subtle changes in their meaning. Were really "free electrons," he was actually disagreeing When the Irish physicist Georgeįrancis FitzGerald suggested in 1897 that Thomson's corpuscles But Larmor's theory did not describe theĮlectron as a part of the atom. Larmor devised a theory of the electron thatĭescribed it as a structure in the ether (the invisible elasticįluid that was proposed as a substrate for light and otherĮlectrical phenomena). That passed electric current through chemicals. Johnstone Stoney in 1891, had been used to denote the unit of charge found in experiments I was even told long afterwards by a distinguished physicist who had been present at my lecture at the Royal Institution that he thought I had been 'pulling their legs.'" Years later he recalled, "At first there were very few who believed in the existence of these bodies smaller than atoms. The second and third hypotheses were especially controversial (the third hypothesis indeed turned out to be false). Homson's speculations met with some skepticism. THOMSON talk about the size of the electron. These corpuscles are the only constituents of the atom.These corpuscles are constituents of the atom.Cathode rays are charged particles (which he called "corpuscles").Homson presented three hypotheses about cathode rays based on his 1897 experiments:
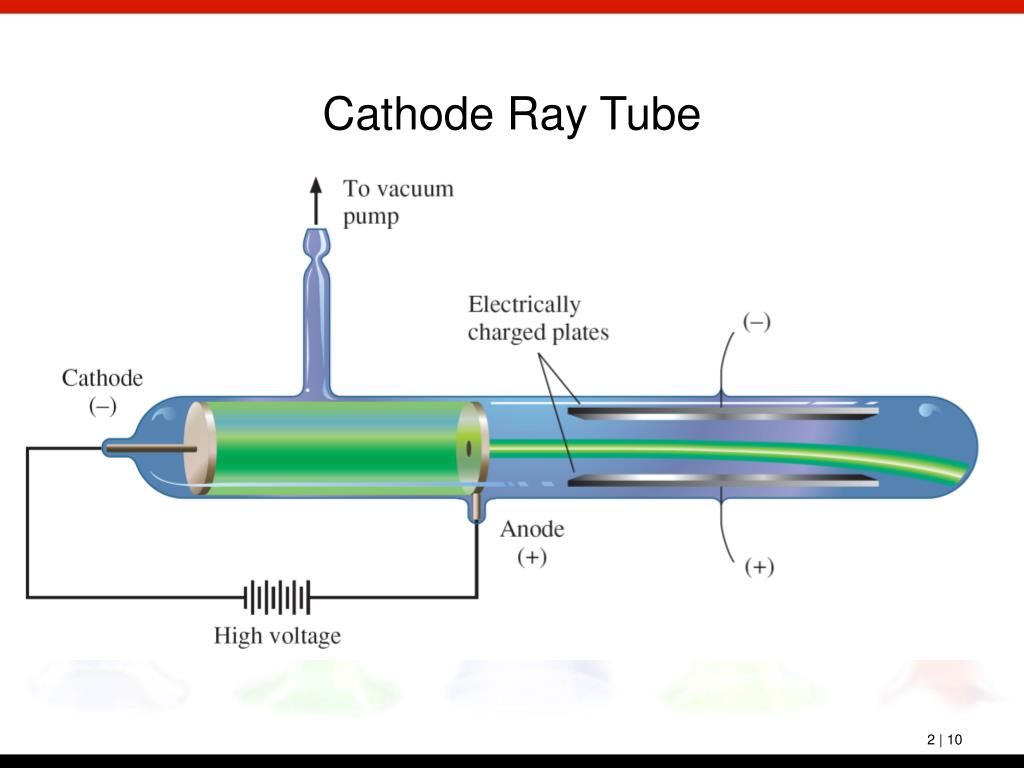
"At first there were very few who believed in the existence of these bodies smaller than atoms." From Thomson's Corpuscles to the Electron


 0 kommentar(er)
0 kommentar(er)
